|
August 02, 2025 NNMT: Key Metabolic‑Epigenetic Regulator, Cancer Target, and Aging Modulator Nicotinamide N‑methyltransferase (NNMT) links nicotinamide metabolism with epigenetic methylation cycles, influences NAD+, mediates cancer cell behavior, and may serve as a biomarker and therapeutic target.
NNMT: Key Metabolic‑Epigenetic Regulator, Cancer Target, and Aging Modulator 1. Introduction Nicotinamide N‑methyltransferase (NNMT) has emerged as a crucial enzyme at the crossroads of metabolism, epigenetics, ageing, and cancer biology. While traditionally regarded as a vitamin B₃ clearance enzyme, over the past decade NNMT’s role has expanded dramatically—to include reprogramming one‑carbon metabolism, impacting NAD⁺ levels, DNA/histone methylation, and even tumor progression and angiogenesis. This article examines NNMT’s enzymatic function, its metabolic–epigenetic influence, and its emerging status as an oncologic biomarker and therapeutic target. 2. Biochemical Function of NNMTNNMT catalyzes the methylation of nicotinamide (vitamin B₃) using S‑adenosyl‑L‑methionine (SAM) as a methyl donor to form 1‑methylnicotinamide (1‑MNAM) and S‑adenosyl‑homocysteine (SAH).
SAM consumption introduces a depletion of methyl donors, directly influencing global cellular methylation potential.
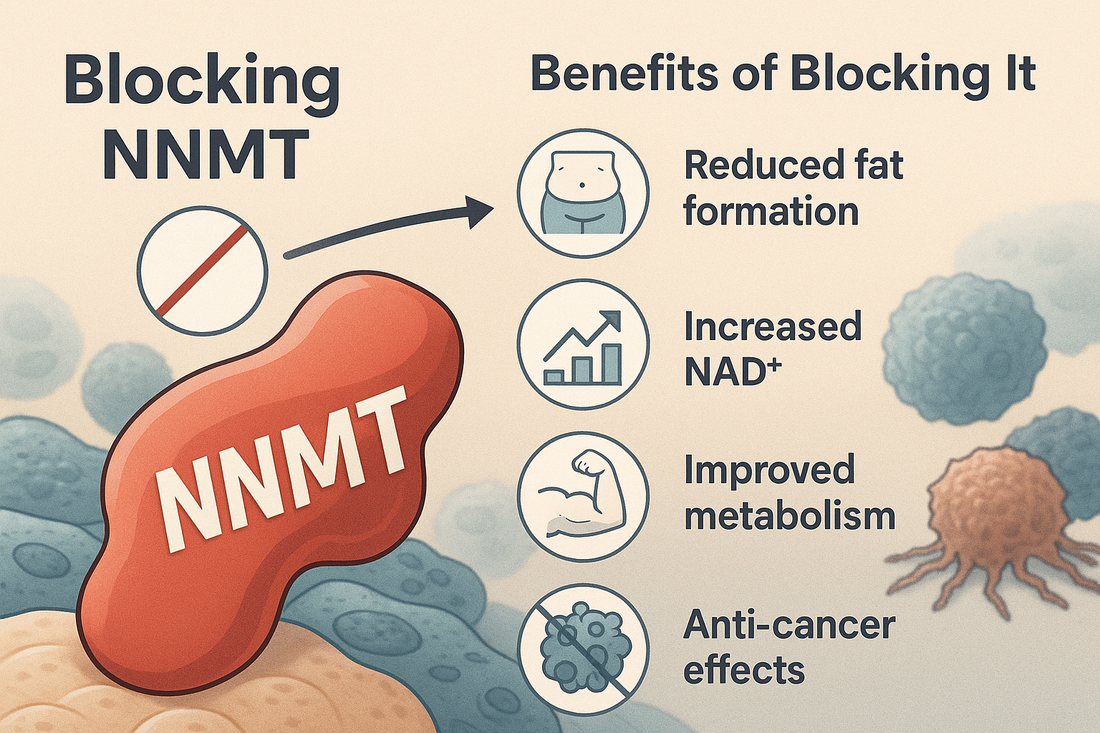
NNMT expression varies widely: liver (where SAM pools are large) vs. adipose or cancer cells (where NNMT overexpression more severely impacts methylation). Through this reaction, NNMT links nicotinamide metabolism with methyl group metabolism, impacting both NAD⁺ salvage pathways and the epigenetic landscape of cells. 3. NNMT and NAD⁺ MetabolismBy methylating nicotinamide, NNMT consumes NAM that would otherwise feed into the salvage pathways for NAD⁺ synthesis, thereby reducing intracellular NAD⁺ levels. NAD⁺ is essential for:
Mitochondrial oxidative phosphorylation
Sirtuin activity (especially SIRT1)
DNA repair and cellular longevity In adipose tissue and certain cancers, high NNMT activity reduces NAD⁺, impairing energy metabolism and promoting insulin resistance. In contrast, in the liver, NNMT–derived 1‑MNAM can actually stabilize SIRT1, potentially exerting beneficial metabolic effects. 4. Epigenetic Impact: The Methyl Sink Phenomenon 4.1 Global HypomethylationNNMT depletes cellular SAM pools, reducing methyl availability for DNA and histone methyltransferases, leading to global hypomethylation in many contexts. This 'methyl sink' effect can:
Alter histone marks (e.g. H3K27 trimethylation)
Derepress oncogenic gene expression
Silence tumor suppressor loci 4.2 Mechanistic Studies
In oral squamous carcinoma-associated fibroblasts, NNMT overexpression reduces H3K27 methylation, enabling ETS2 transcription, which then increases VEGFA expression and promotes angiogenesis.
More broadly, epigenetic dysregulation via NNMT influences polyamine biosynthesis and cell proliferation. 5. NNMT in Cancer: Biomarker and Driver 5.1 Overexpression and PrognosisNNMT is upregulated in many solid tumors—including breast, lung, colon, pancreatic and head & neck cancers—and high levels often correlate with poor overall survival and chemoresistance. Pan‑cancer analyses confirm:
NNMT expression correlates with tumor mutational burden (TMB) and microsatellite instability (MSI).
Its expression associates with infiltration of immune cells—suggesting a role in shaping the tumor microenvironment. 5.2 Functional Oncogenic Roles
NNMT promotes proliferation, migration, invasion, and supports the Warburg phenotype in cancer cells.
In cancer-associated fibroblasts, NNMT drives angiogenesis through the ETS2–VEGFA axis via epigenetic reprogramming. Knockdown or inhibition of NNMT in experimental models reduces tumor growth, metastasis, and restores sensitivity to therapies (e.g. overcoming EGFR‑TKI resistance in lung cancer cells). 6. Therapeutic Targeting of NNMT 6.1 Inhibitor DevelopmentMultiple NNMT inhibitors—including natural compounds like yuanhuadine—have shown promise in sensitizing cancer cells to existing therapies and reducing proliferation. 5-Amino-1MQ is one such compound. 6.2 Antisense Oligonucleotide (ASO) ApproachesASOs targeting NNMT mRNA in liver and adipose tissue have been studied preclinically, showing improvements in insulin sensitivity and reduction of obesity phenotypes without significant toxicity. 6.3 Epigenetic Reprogramming StrategyGiven NNMT’s role in the epigenetic architecture of tumor stroma, targeting it may help overcome therapy resistance—specifically in cancers where tumor angiogenesis and fibrosis are driven by NNMT‑expressing fibroblasts. 7. NNMT in Aging and Metabolic Disease 7.1 Obesity, Insulin Resistance, NAFLD/NASHHigh NNMT expression in adipose tissue correlates with obesity, insulin resistance, and progression of non‑alcoholic fatty liver disease to NASH, due to decreased NAD⁺ and global methyl depletion. NNMT knockdown in animal studies protects against weight gain on high-fat diets—even without dietary or activity changes. 7.2 Stem Cells & LongevityNNMT expression maintains stem‑like identity in embryonic stem cells, partly via its effect on methylation and metabolic state. In model organisms, modulation of NNMT products (e.g. 1‑MNAM) enhances SIRT1 activity and may influence lifespan and redox balance. 8. Epigenetics Basics: How NNMT Fits InEpigenetics refers to heritable changes in gene expression that do not involve alterations in DNA sequence—commonly involving DNA methylation, histone modification, or non‑coding RNA regulation. NNMT influences this regulatory landscape via:
Reducing SAM availability for DNA and histone methylation
Causing altered chromatin states that can activate oncogenes or silence tumor suppressors
Facilitating oncogenic reprogramming in both tumor cells and stromal microenvironment 9. Summary – Why NNMT Matters
Metabolic hub: Couples nicotinamide clearance with SAM use and NAD⁺ regulation.
Epigenetic modulator: Alters methylation status across genes and histones.
Oncogenic driver: Supports cancer cell survival, angiogenesis, invasion, and therapy resistance.
Biomarker & target: Elevated in many cancers; targeting NNMT yields anti‑tumor effects and may reverse resistance.
Aging & metabolic disease link: Implicated in obesity, insulin resistance, fatty liver, stem cell function, and lifespan regulation. 10. Future Directions & Clinical Applications 10.1 Inhibitor DiscoveryNext-gen NNMT inhibitors—small molecules, ASOs, or siRNAs—are under preclinical development with encouraging results. 10.2 Precision OncologyNNMT expression profiling may guide prognosis, predict therapy responsiveness, or support combination strategies with anti-angiogenic or epigenetic drugs in cancers such as OSCC, bladder cancer, pancreatic, or lung carcinoma. 10.3 Metabolic Disease & LongevityModulating NNMT function or expression could become a strategy to preserve NAD⁺ during aging, improve metabolic resilience, and delay epigenetic ageing. 11. Clinical & Research Considerations
Measure tissue-specific effects: In liver vs. adipose vs. tumor, NNMT behaves differently.
Post-translational regulation of NNMT (e.g., phosphorylation, succinylation) adds complexity and may be targeted in future therapies.
Longitudinal monitoring: Effects on methylation potential and NAD⁺ may evolve over years.
Safety and off-target effects: Especially for systemic inhibitors intended to modulate global methylation. Call to ActionIf you're interested in how NNMT regulation can be leveraged for better metabolic health, cancer diagnostics, or therapeutic innovation, our team offers strategic research partnerships and custom clinical biomarker programs. Let us help translate NNMT biology into transformative outcomes. 📚 Scientific References
Küçükkaraca S., et al. Nicotinamide N‑methyltransferase: A primarily cytoplasmic methyltransferase expressed in liver and overexpressed in cancers. Sci Direct. 2024. (ScienceDirect)
Parsons RB & Facey PD. Nicotinamide N‑Methyltransferase: an emerging protagonist in cancer macro(r)evolution. Biomolecules. 2021. (MDPI)
Frontiers in Oncology review: NNMT as a biomarker, therapeutic target, and epigenetic regulator. Front Oncol. 2022. (Frontiers, Frontiers)
Study on NNMT-mediated tumor angiogenesis via ETS2–VEGFA axis (OSCC context). Nat. Oncol. 2024. (Nature)
Comprehensive pan-cancer analysis: NNMT expression, prognosis, immune infiltration, and epigenetic associations. Frontiers Genetics. 2022. (Frontiers)
Antisense oligonucleotide targeting NNMT in metabolic tissues: effects on methylation and metabolic syndrome. Sci Direct. 2025. (ScienceDirect)
Methyl‑sink and polyamine biogenesis roles of NNMT in cell aging and proliferation. Springer Pharmacol Sci. 2024. (SpringerLink)
Enzymology review: kinetic constants, post‑translational modifications, and regulation. Cell Death Differ. 2022. (Nature, ScienceDirect)
Overview of NAM methylation, NAD⁺ metabolism, and NNMT structural biology. Wikipedia / biochemical sources. 2023 – 2024. (Wikipedia, Wikipedia, Wikipedia)
Introductory definitions of epigenetics & cancer epigenetics mechanisms. Wikipedia. 2024. (Wikipedia) (责任编辑:) |